MIDAA 101 (on 10X multiome)
10x Multiomics analysis
As our first tutorial we will use the CD34+ positive 10X ATAC + GEX multiome dataset from Setty et al.. The dataset
import sys
sys.path.append("/home/salvatore.milite/work/python_packages/daario/src/")
%load_ext autoreload
%autoreload 2
The autoreload extension is already loaded. To reload it, use:
%reload_ext autoreload
# you need some extra packages to run the tutorial you can find the list on tutorial_requirements.txt
import midaa as maa
import torch
import numpy as np
import scanpy as sc
import pandas as pd
import matplotlib.pyplot as plt
# I like snapatac but you can you use whatever you want for preprocessing atac
import snapatac2 as snap
import muon as mu
import mudata
from muon import atac as ac
from pyjaspar import jaspardb
import pychromvar as pc
# sorry I'm kinda in love with ggplot
from plotnine import ggplot, geom_point, aes, stat_smooth, facet_wrap, xlim, theme_classic, theme, ggtitle, ggsave
%matplotlib inline
import matplotlib
matplotlib.rcParams['figure.figsize'] = [4.5, 4.5]
matplotlib.rcParams['figure.dpi'] = 90
The data can be obtained from the server of the original authors:
# !mkdir data/
# !wget https://dp-lab-data-public.s3.amazonaws.com/SEACells-multiome/cd34_multiome_rna.h5ad -O data/cd34_multiome_rna_processed.h5ad # RNA data
# !wget https://dp-lab-data-public.s3.amazonaws.com/SEACells-multiome/cd34_multiome_atac.h5ad -O data/cd34_multiome_atac.h5ad # ATAC data
ad_atac = sc.read('./data/cd34_multiome_atac.h5ad')
ad_rna = sc.read('./data/cd34_multiome_rna_processed.h5ad')
sc.pl.umap(ad_rna, color="celltype")
/home/salvatore.milite/miniconda3/envs/scdeepaa/lib/python3.11/site-packages/scanpy/plotting/_tools/scatterplots.py:394: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap' will be ignored
cax = scatter(
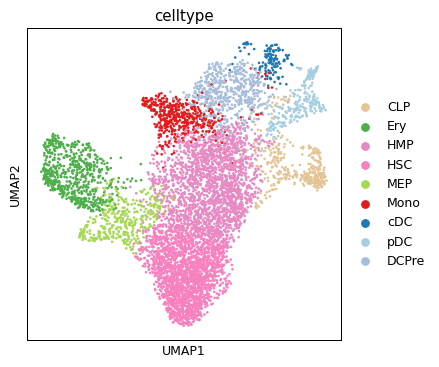
sc.pl.umap(ad_atac, color="celltype")
/home/salvatore.milite/miniconda3/envs/scdeepaa/lib/python3.11/site-packages/scanpy/plotting/_tools/scatterplots.py:394: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap' will be ignored
cax = scatter(
We will first run a bit of very fast and rough pre-processing on the atac dataset
# We will make the dataset a little bit more manageble by
# using a subset of highly variable peaks
ad_atac_hv = ad_atac
snap.pp.select_features(ad_atac, n_features=10000)
# We do the same for RNA, but you can fit the model on the
# whole dataset, this is mainly for a matter of efficiency
ad_rna_hv = ad_rna[:,ad_rna.var["highly_variable"]].copy()
ad_atac_hv = ad_atac[:,ad_atac.var["selected"]].copy()
ad_atac_hv.raw = ad_atac_hv
2024-04-12 15:31:13 - INFO - Selected 10000 features.
# Scale and normalize so we cna use a Gaussian likelihood
sc.pp.scale(ad_rna_hv)
sc.pp.normalize_total(ad_atac_hv)
sc.pp.log1p(ad_atac_hv)
sc.pp.scale(ad_atac_hv)
We need a minimum of 3 inputs types to run MIDAA: the actual data matrices, an optional normalization factor and a likelihood type. We have a simple parser function for AnnData objects but it is always better to understand how to do it explicitly:
input_data = [ad_rna_hv.X, ad_atac_hv.X]
normalization = [np.ones(ad_rna_hv.X.shape[0]), np.ones(ad_atac_hv.X.shape[0])] # If you have a normalization factor for each cell, you can provide it here, we used scaled data so no need for that
input_distribution = ["G", "G"] # G for Gaussian, P for Poisson, NB for Negative Binomial, B for Bernoulli, Beta for Beta distribution and C for Categorical
# As a quick note, as you see MIDAA is developed for multi-modal data,
# so even if you use it for a single modality the inputs are always lists.
We will now run a for loop to test different models (depending on what is you final goal you might even fix the number of archetypes to be in the range 5-10 as this could probably be enough, or maybe if you already know how many extreme groups you have):
# A way to increase the network size fast
MULT = 4
models = {}
# This takes around 15 minutes on a NVIDIA V100 32GB
for i in range(2,12):
models[i] = maa.fit_MIDAA(
input_data,
normalization,
input_distribution,
hidden_dims_dec_common = [64 * MULT, 128 * MULT], # Hidden dimensions of the common decoder
hidden_dims_dec_last = [256 * MULT], # Hidden dimensions of the modality specific decoder
hidden_dims_enc_ind = [128 * MULT], # Hidden dimensions of the modality specific encoder
hidden_dims_enc_common = [64 * MULT], # Hidden dimensions of the common encoder
hidden_dims_enc_pre_Z = [32 * MULT], # Hidden dimensions of the matrix specific encoder
lr = 0.0001, # Learning rate
gamma_lr = 0.1, # Learning rate decay factor
steps = 1000,
narchetypes = i,
batch_size = 9000,
torch_seed = 3
)
ELBO: 411358432.00000 : 100%|██████████| 1000/1000 [01:56<00:00, 8.62it/s]
/home/salvatore.milite/miniconda3/envs/scdeepaa/lib/python3.11/site-packages/pyro/primitives.py:137: RuntimeWarning: trying to observe a value outside of inference at loss
warnings.warn(
ELBO: 411193952.00000 : 100%|██████████| 1000/1000 [01:55<00:00, 8.62it/s]
ELBO: 411180704.00000 : 100%|██████████| 1000/1000 [01:56<00:00, 8.61it/s]
ELBO: 411094816.00000 : 100%|██████████| 1000/1000 [01:56<00:00, 8.62it/s]
ELBO: 411099968.00000 : 100%|██████████| 1000/1000 [01:56<00:00, 8.61it/s]
ELBO: 411067808.00000 : 100%|██████████| 1000/1000 [01:55<00:00, 8.63it/s]
ELBO: 411077184.00000 : 100%|██████████| 1000/1000 [01:55<00:00, 8.63it/s]
ELBO: 411017408.00000 : 100%|██████████| 1000/1000 [01:55<00:00, 8.63it/s]
ELBO: 410968736.00000 : 100%|██████████| 1000/1000 [01:59<00:00, 8.40it/s]
ELBO: 411006688.00000 : 100%|██████████| 1000/1000 [01:55<00:00, 8.62it/s]
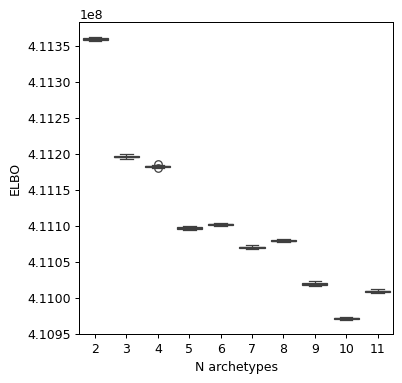
We see how 5 is a potential candidate for a representative group of archetypes, then we have another drop of the loss at 10 which can also be used for a more refined analysis. We will go for 5 in this tutorial, but remember to explore more solutions.
maa.plot_ELBO_across_runs(models, 950) # warm-up 950 epochs
2024-04-12 16:42:17 - INFO - Using categorical units to plot a list of strings that are all parsable as floats or dates. If these strings should be plotted as numbers, cast to the appropriate data type before plotting.
2024-04-12 16:42:17 - INFO - Using categorical units to plot a list of strings that are all parsable as floats or dates. If these strings should be plotted as numbers, cast to the appropriate data type before plotting.
Let’s first save the model as the computation is quite expensive!
best_model = res[5]
torch.save(best_model["deepAA_obj"].state_dict(), "best_model_aa_state_dict.pth")
torch.save(best_model["inferred_quantities"], "best_model_aa_inferred_quantities.pth")
# We can also save the ELBO values for this run
torch.save({k:v["ELBO"] for k,v in models.items()}, "ELBO_dict.pth")
# To reload the model in the future you initialize it with the same parameters
best_model = maa.fit_MIDAA(
input_data,
normalization,
input_distribution,
hidden_dims_dec_common = [64 * MULT, 128 * MULT], # Hidden dimensions of the common decoder
hidden_dims_dec_last = [256 * MULT], # Hidden dimensions of the modality specific decoder
hidden_dims_enc_ind = [128 * MULT], # Hidden dimensions of the modality specific encoder
hidden_dims_enc_common = [64 * MULT], # Hidden dimensions of the common encoder
hidden_dims_enc_pre_Z = [32 * MULT], # Hidden dimensions of the matrix specific encoder
lr = 0.0001, # Learning rate
gamma_lr = 0.1, # Learning rate decay factor
steps = 1,
narchetypes = 5,
batch_size = 9000,
torch_seed = 3
)
# and then run
maa.load_model_from_state_dict(best_model,input_data, "best_model_aa_state_dict.pth") # you need the initialized model, the same input and the state dict file
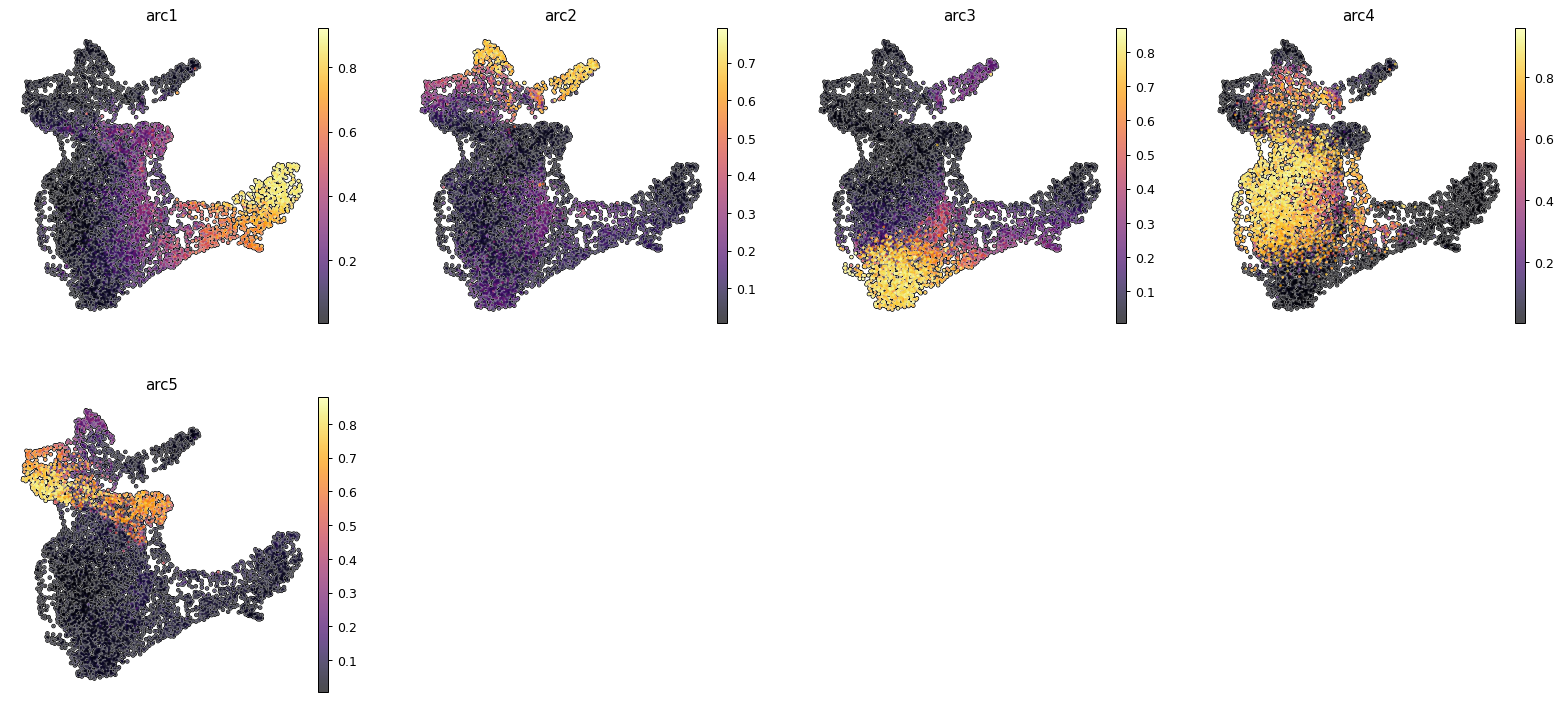
After this digression we can go on with our analysis and try to visualize the archetypes results. We can see how archetype follow mostly the erythroid lineage, with archetype 2,3 and 5 being related to Dendritic, stem and monocyte lines. Archetype 4 is still mostly in the transitioning stem cell portion of the dataset.
# Utility functions to add archetypes weights to the anndata object
ad_rna, arc_names = maa.add_to_obs_adata(best_model, ad_rna)
ad_atac, arc_names = maa.add_to_obs_adata(best_model, ad_atac)
sc.pl.umap(ad_rna, color=arc_names, cmap = "inferno", frameon = False, add_outline=True)
/home/salvatore.milite/miniconda3/envs/scdeepaa/lib/python3.11/site-packages/scanpy/plotting/_tools/scatterplots.py:371: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap', 'norm' will be ignored
ax.scatter(
/home/salvatore.milite/miniconda3/envs/scdeepaa/lib/python3.11/site-packages/scanpy/plotting/_tools/scatterplots.py:381: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap', 'norm' will be ignored
ax.scatter(
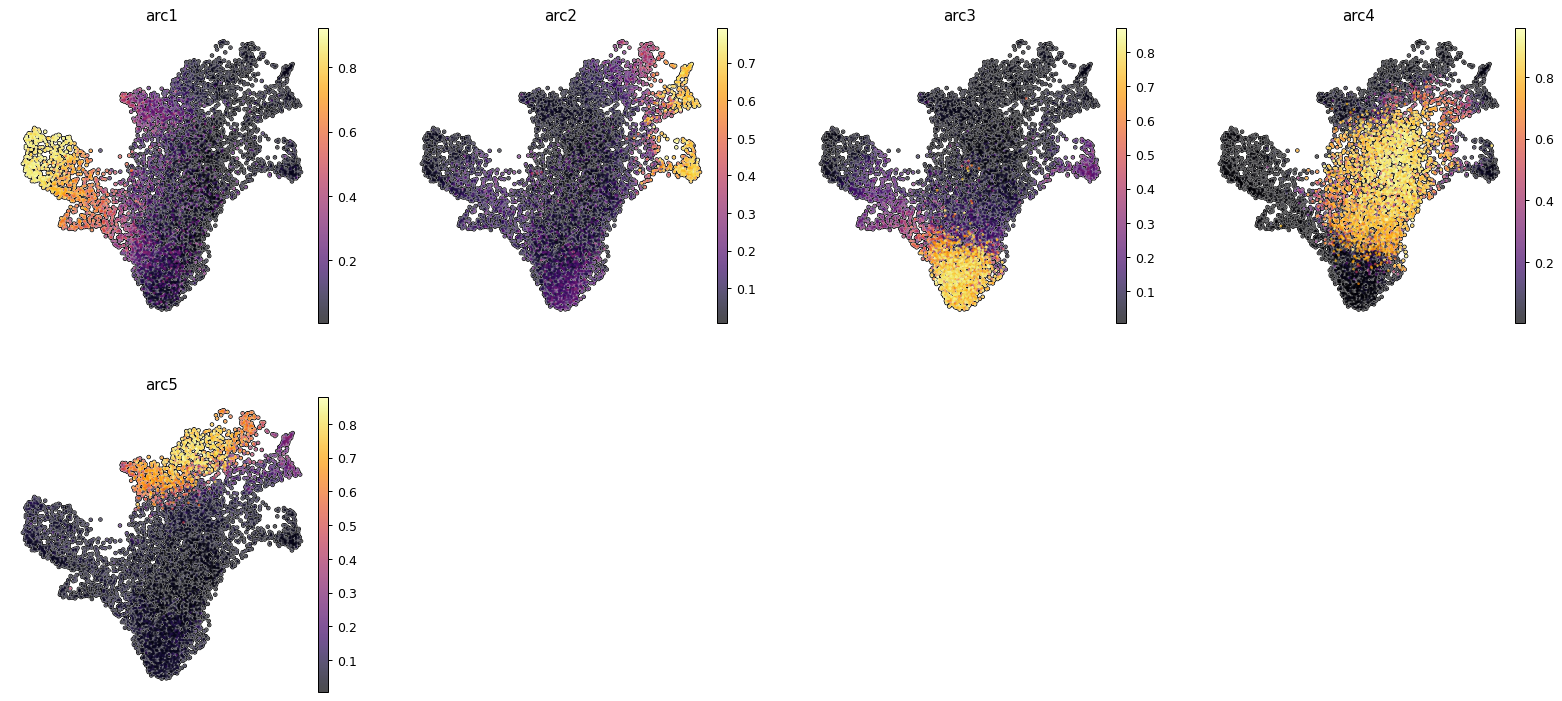
sc.pl.umap(ad_atac, color=arc_names, cmap = "inferno", frameon = False, add_outline=True)
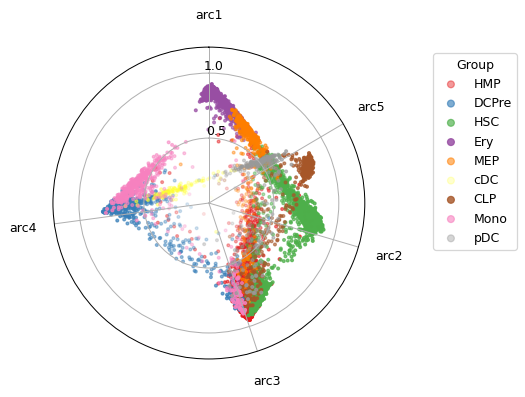
Another way of visualizing MIDAA is to plot a 2d projection of the latent space on a polytope (this visualization is quite reflective of the latent space even though it gets a bit hard to understand if you have a lot of archetypes)
maa.plot_archetypes_simplex(best_model, s = 5, color_by=ad_rna.obs["celltype"], cmap = "Set1")
(<Figure size 405x405 with 1 Axes>, <PolarAxes: >)
Then we can exploit the chromatin to look at some transcription factors involved in the differentiation and look at how they correlate with the archetypes. To do so we will first compute TF deviations using pychromVAR
mdata = mudata.MuData({"rna": ad_rna, "atac": ad_atac})
# For more information you can look at the pychromVAR documentation (https://pychromvar.readthedocs.io/en/latest/)
pc.get_genome("hg38", output_dir="./")
pc.add_peak_seq(mdata, genome_file="./hg38.fa", delimiter=":|-")
pc.add_gc_bias(mdata)
pc.get_bg_peaks(mdata)
# get motifs
jdb_obj = jaspardb(release='JASPAR2020')
motifs = jdb_obj.fetch_motifs(
collection = 'CORE',
tax_group = ['vertebrates'])
pc.match_motif(mdata, motifs=motifs)
dev = pc.compute_deviations(mdata)
dev.write_h5ad("data/chromvar_deviations.h5ad")
ELBO: 9907476.00000 : 100%|██████████| 250/250 [00:13<00:00, 18.85it/s]
/home/salvatore.milite/miniconda3/envs/scdeepaa/lib/python3.11/site-packages/pyro/primitives.py:137: RuntimeWarning: trying to observe a value outside of inference at loss
warnings.warn(
ELBO: 9901263.00000 : 100%|██████████| 250/250 [00:13<00:00, 19.10it/s]
ELBO: 9899080.00000 : 100%|██████████| 250/250 [00:13<00:00, 18.79it/s]
ELBO: 9892848.00000 : 100%|██████████| 250/250 [00:13<00:00, 18.89it/s]
ELBO: 9889298.00000 : 100%|██████████| 250/250 [00:13<00:00, 18.90it/s]
ELBO: 9889507.00000 : 100%|██████████| 250/250 [00:13<00:00, 19.03it/s]
ELBO: 9892396.00000 : 100%|██████████| 250/250 [00:13<00:00, 19.02it/s]
ELBO: 9888861.00000 : 100%|██████████| 250/250 [00:13<00:00, 19.05it/s]
ELBO: 9884211.00000 : 100%|██████████| 250/250 [00:13<00:00, 19.13it/s]
ELBO: 9881814.00000 : 100%|██████████| 250/250 [00:13<00:00, 19.07it/s]
dev = sc.read_h5ad("data/chromvar_deviations.h5ad")
# We are again moving stuff around for plotting
mdata.mod['chromvar'] = dev
mdata.mod['chromvar'].raw = dev
mdata["chromvar"].obsm["X_umap"] = mdata["rna"].obsm["X_umap"]
mdata.obsm["rna_umap"] = mdata["rna"].obsm["X_umap"]
mdata.obsm["atac_umap"] = mdata["atac"].obsm["X_umap"]
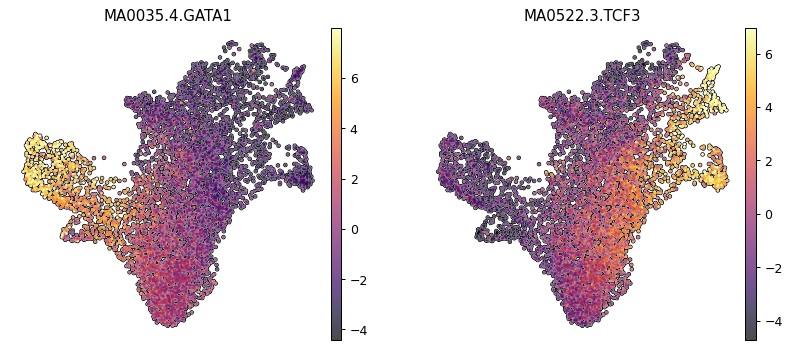
We will pick as example GATA1 and TCF3 which are important in respectively erythroid and dendritic differentiation. We can also see how their expression is quite low.
sc.pl.umap(mdata["chromvar"], color=['MA0035.4.GATA1', "MA0522.3.TCF3"], cmap = "inferno", frameon = False, add_outline=True, vmin='p1', vmax='p99')
/home/salvatore.milite/miniconda3/envs/scdeepaa/lib/python3.11/site-packages/scanpy/plotting/_tools/scatterplots.py:371: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap', 'norm' will be ignored
ax.scatter(
/home/salvatore.milite/miniconda3/envs/scdeepaa/lib/python3.11/site-packages/scanpy/plotting/_tools/scatterplots.py:381: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap', 'norm' will be ignored
ax.scatter(
sc.pl.umap(mdata["rna"], color=['GATA1', "TCF3"], cmap = "inferno", frameon = False, add_outline=True, vmin='p1', vmax='p99')
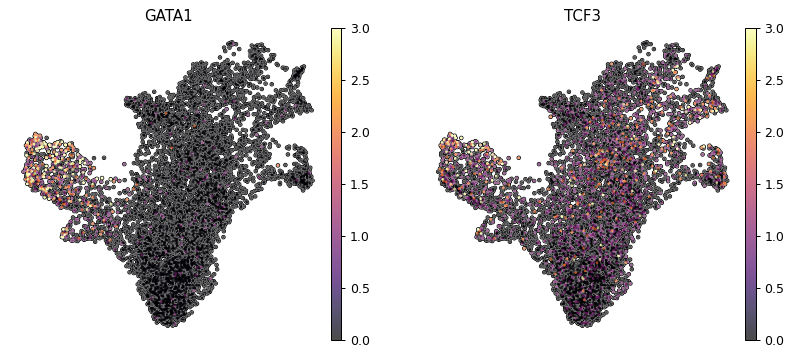
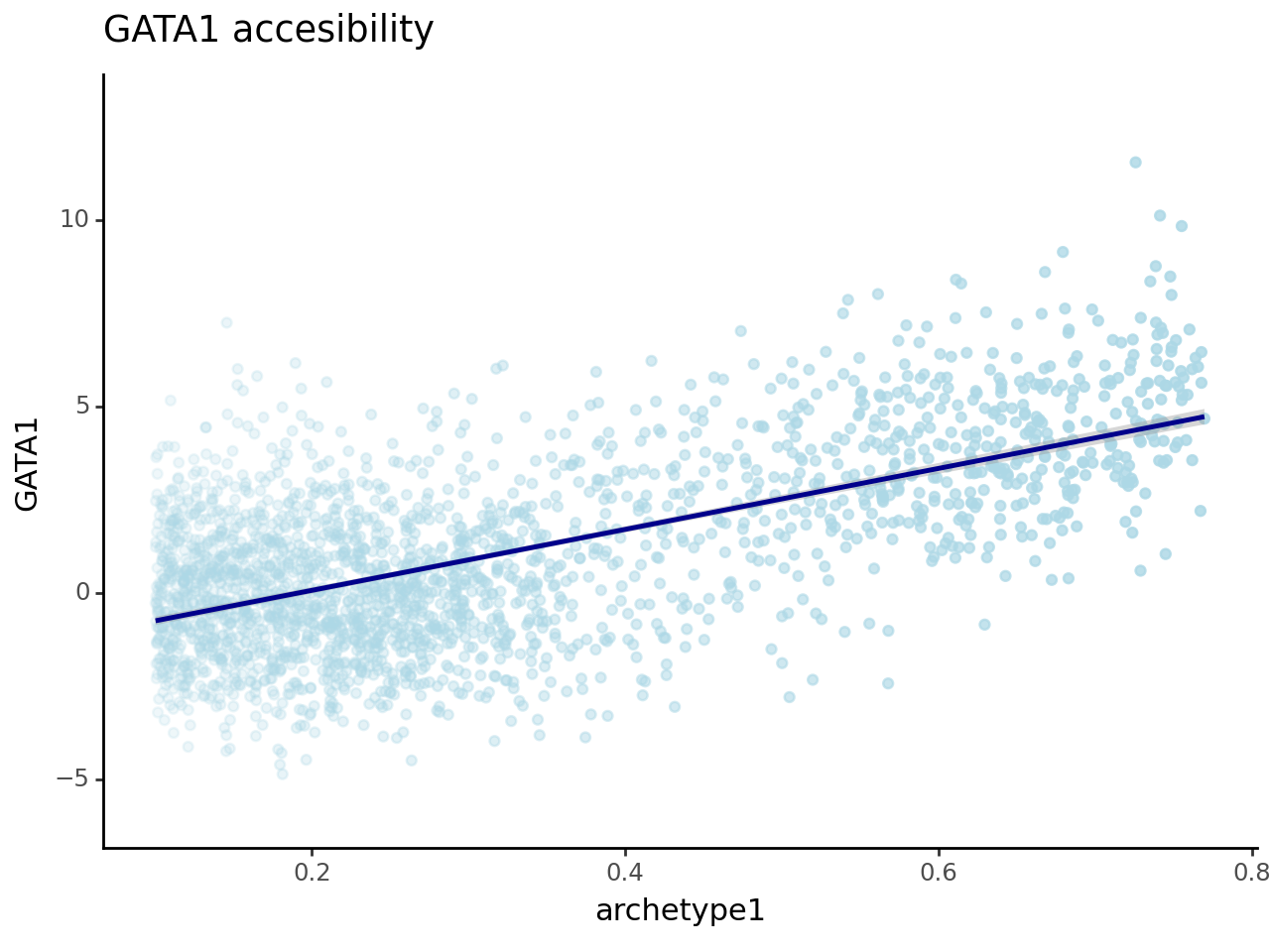
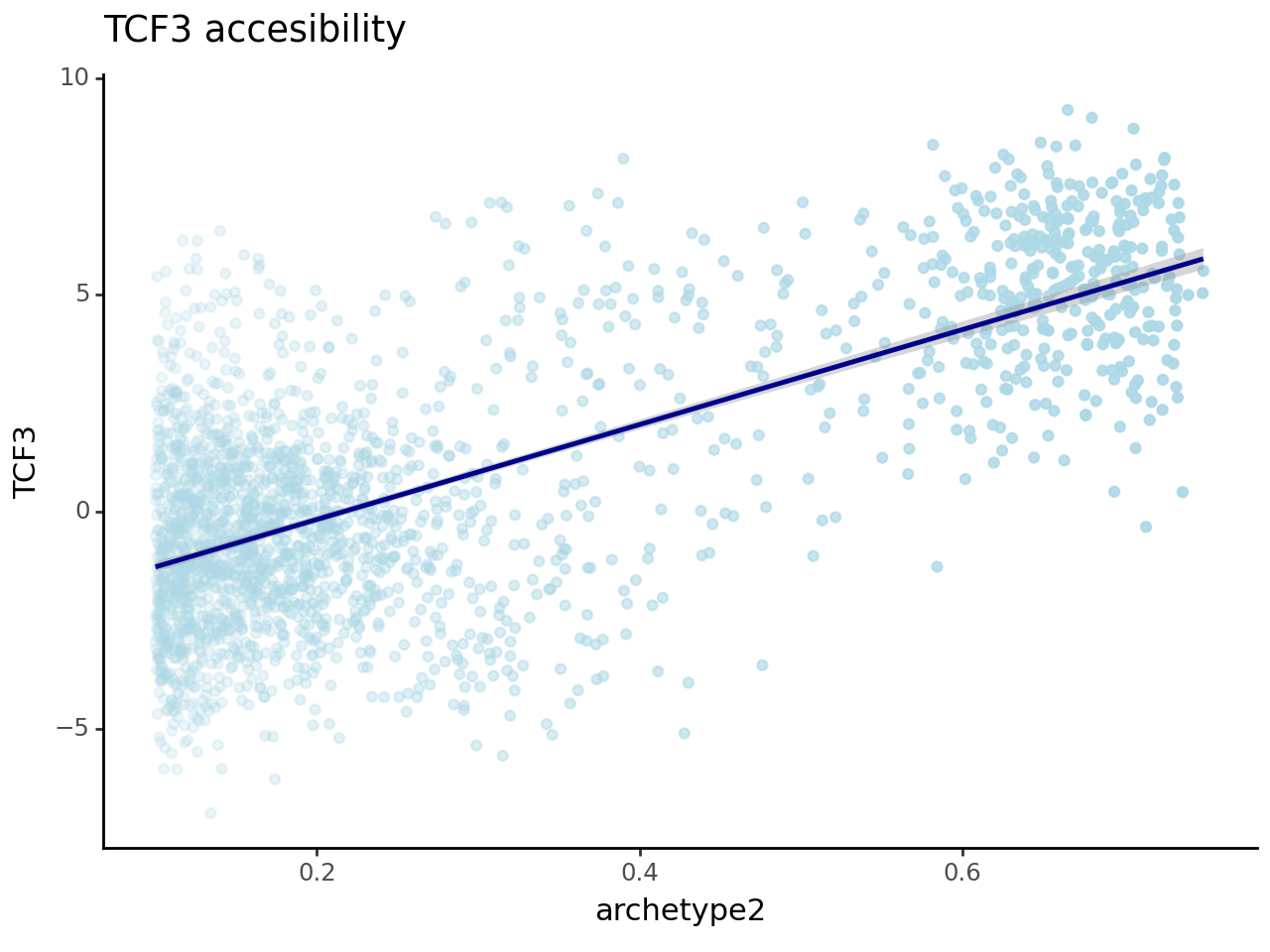
We now want to correlate the TF score to the archetypes weight, so we take archetypes 1 and 2 as we saw they were recapitulating erythroid and dendritic lineages.
df_plot_1 = pd.DataFrame({
"TCF3" : np.array(mdata["chromvar"][:,"MA0522.3.TCF3"].X).flatten(),
"modality" : "atac",
"archetype2" : mdata["rna"].obs["arc2"]
}, index = mdata["rna"].obs.index)
df_plot_2 = pd.DataFrame({
"GATA1" : np.array(mdata["chromvar"][:,"MA0035.4.GATA1"].X).flatten(),
"modality" : "atac",
"archetype1" : mdata["rna"].obs["arc1"]
}, index = mdata["rna"].obs.index)
And indeed we have a pretty satisfying correlation, which hints at the fact that maybe we did not waste the last hour but we are actually extracting some biology here.
(
ggplot(df_plot_2, aes("archetype1", "GATA1", alpha="archetype1"))
+ geom_point(color = "lightblue")
+ stat_smooth(color = "darkblue") +
xlim([0.1,0.77]) + theme_classic() + theme(legend_position = "none") +
ggtitle("GATA1 accesibility")
)
/home/salvatore.milite/miniconda3/envs/scdeepaa/lib/python3.11/site-packages/plotnine/layer.py:364: PlotnineWarning: geom_point : Removed 4449 rows containing missing values.
<Figure Size: (640 x 480)>
(
ggplot(df_plot_1, aes("archetype2", "TCF3", alpha="archetype2"))
+ geom_point(color = "lightblue")
+ stat_smooth(color = "darkblue") +
xlim([0.1,0.75]) + theme_classic() + theme(legend_position = "none") +
ggtitle("TCF3 accesibility")
)
/home/salvatore.milite/miniconda3/envs/scdeepaa/lib/python3.11/site-packages/plotnine/layer.py:364: PlotnineWarning: geom_point : Removed 4473 rows containing missing values.
<Figure Size: (640 x 480)>
Once you have the archetypes you can do a lot of stuff with them, by using them as if they were proper sample :
You can GSEA on their expression profile
You can look at how they evolve over space
You can compute enrichment of features across one archetype weights
You can use archetype weights as covariates in a survival model
Imagination is you only limit!
Generating new data
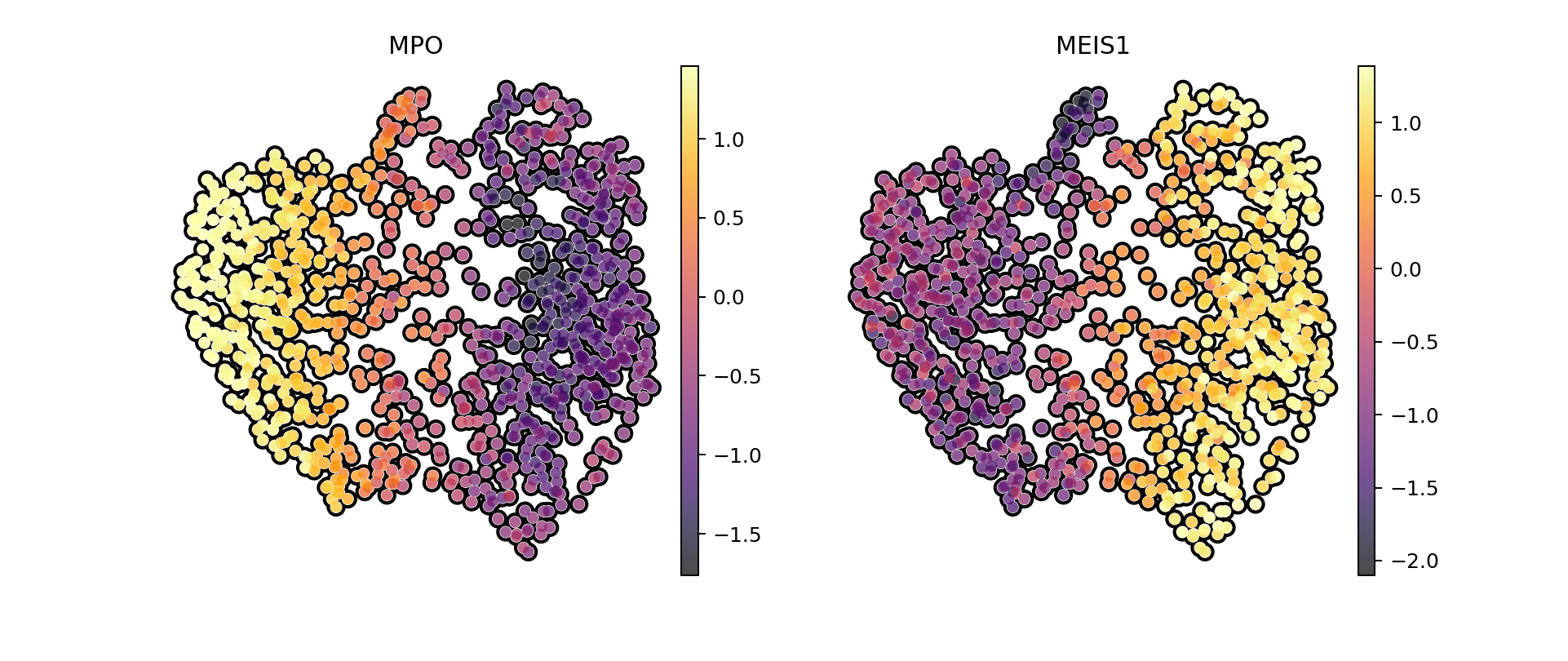
But want I would like to show in this last part of the tutorial which is less straightforward to use is how we can use our model in a generative fashion to simulate synthetic data. The idea is that we specify some coordinates in the simplex and then we sample from teh corresponding Dirichlet distribution. For instance in this example here we will sample a dataste mostly composed by 2 archetypes
AA_distribution = torch.tensor([1e-16,1e-16, 2,0.1, 2]).cpu()
# We have a custom function for this, just give the concentration of a Dirichlet distribution or a full A matrix
synthetic_matrices, _ = maa.generate_synthetic_data(best_model, AA_distribution, seed = 6, to_cpu = True)
We then treat it as a normal single cell experiment and try to look at some markers in ATAC and RNA space
import anndata as ad
cell_names = ["Cell_" + str(i) for i in range(synthetic_matrices[0].shape[0])]
ad_synt_rna = ad.AnnData(synthetic_matrices[0].detach().numpy())
ad_synt_rna.obs_names = cell_names
ad_synt_rna.var_names = ad_rna_hv.var_names
sc.pp.scale(ad_synt_rna)
sc.tl.pca(ad_synt_rna)
sc.pp.neighbors(ad_synt_rna, )
sc.tl.umap(ad_synt_rna )
# MPO is a stem marker and MEIS1 a dendritic one
sc.pl.umap(ad_synt_rna, color=[ "MPO", "MEIS1"], save = "umap_rna_markers_synthetic.pdf", cmap = "inferno", frameon = False, add_outline=True, vmax = "p95")
WARNING: saving figure to file figures/umapumap_rna_markers_synthetic.pdf
/home/salvatore.milite/miniconda3/envs/scdeepaa/lib/python3.11/site-packages/scanpy/plotting/_tools/scatterplots.py:371: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap', 'norm' will be ignored
/home/salvatore.milite/miniconda3/envs/scdeepaa/lib/python3.11/site-packages/scanpy/plotting/_tools/scatterplots.py:381: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap', 'norm' will be ignored
import anndata as ad
ad_synt_atac = ad.AnnData(synthetic_matrices[1].detach().numpy())
ad_synt_atac.obs_names = cell_names
ad_synt_atac.var_names = ad_atac_hv.var_names
ad_synt_atac.var = ad_atac_hv.var
sc.pp.scale(ad_synt_atac)
# We look at the gene promoters
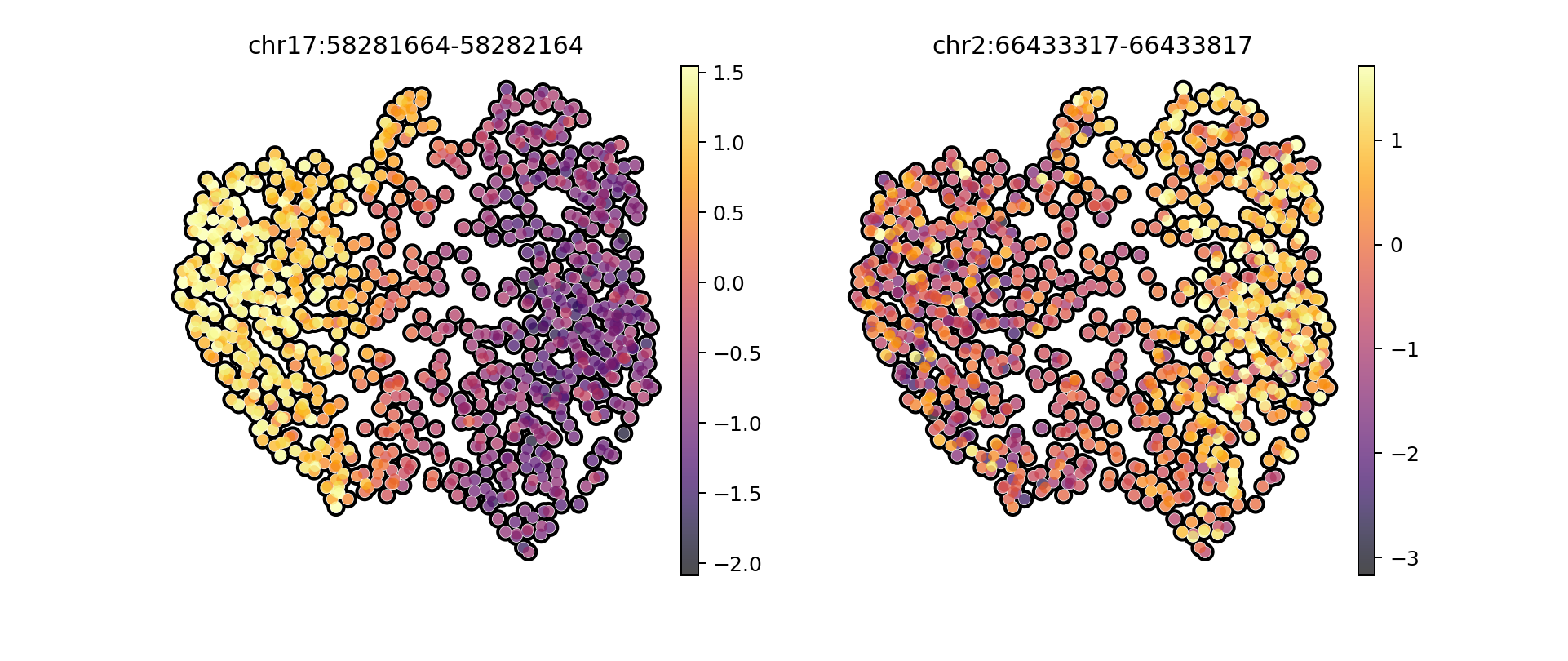
ad_atac_hv.var[ad_atac_hv.var["nearestGene"] == "MPO"][["distToGeneStart", "peakType"]]
| distToGeneStart | peakType | |
|---|---|---|
| chr17:58281664-58282164 | 12058 | Promoter |
ad_atac_hv.var[ad_atac_hv.var["nearestGene"] == "MEIS1"][["distToGeneStart", "peakType"]]
| distToGeneStart | peakType | |
|---|---|---|
| chr2:66433317-66433817 | 115 | Promoter |
| chr2:66434847-66435347 | 1645 | Promoter |
| chr2:66481155-66481655 | 47953 | Intronic |
ad_synt_atac.obsm["X_umap"] = ad_synt_rna.obsm["X_umap"]
sc.pl.umap(ad_synt_atac, color = [ "chr17:58281664-58282164", "chr2:66433317-66433817"], save = "umap_atac_markers_synthetic.pdf", cmap = "inferno", frameon = False, add_outline=True, vmax = "p95")
WARNING: saving figure to file figures/umapumap_atac_markers_synthetic.pdf
/home/salvatore.milite/miniconda3/envs/scdeepaa/lib/python3.11/site-packages/scanpy/plotting/_tools/scatterplots.py:371: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap', 'norm' will be ignored
/home/salvatore.milite/miniconda3/envs/scdeepaa/lib/python3.11/site-packages/scanpy/plotting/_tools/scatterplots.py:381: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap', 'norm' will be ignored